Metro accepts BlueCross BlueShield healthcare plans as an in-network provider. Learn More
 Pediatric First Aid Basics: What Every Caregiver Should Know
Pediatric First Aid Basics: What Every Caregiver Should Know
The Importance of Pediatric First Aid Medical emergencies are unpredictable. When they involve our children, the emotional toll can spike... Read More ›
 How to Manage Allergies in Children: From Food to Pollen
How to Manage Allergies in Children: From Food to Pollen
The Importance of Understanding Allergies in Children Allergies in children are not just a mere inconvenience; they represent how the... Read More ›
 Most Common Symptoms of an Ear Infection
Most Common Symptoms of an Ear Infection
Ear infections are among the most common ailments where parents bring their child to the doctor. It helps to understand... Read More ›
 Metro Accepts BlueCross BlueShield Health Insurance
Metro Accepts BlueCross BlueShield Health Insurance
Notice to Metro Pediatrics Patients Metro is contracted with BlueCross BlueShield as your in-network provider and welcomes all patients with... Read More ›
 Recognizing and Supporting Mental Health in Children
Recognizing and Supporting Mental Health in Children
The Importance of Mental Health in Children As parents, educators, and caregivers, we work together to ensure the wellbeing of... Read More ›
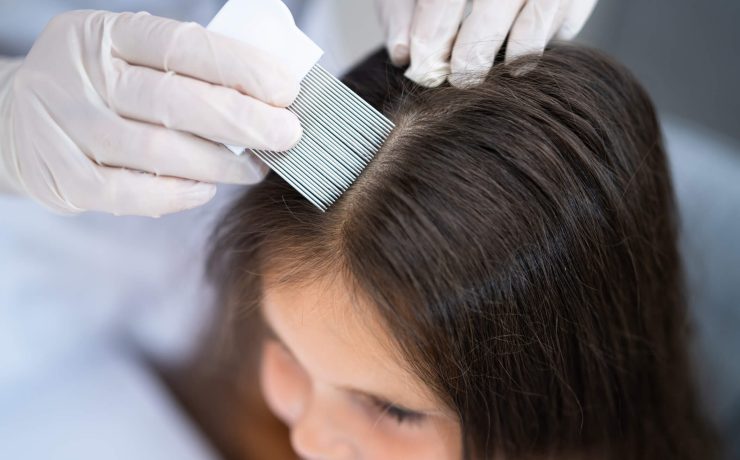 How to Get Rid of Head Lice
How to Get Rid of Head Lice
Head lice. Is it cause for real concern? For some, there’s an “ick” factor associated with a head of lice... Read More ›
 How to Recognize Common Childhood Illnesses
How to Recognize Common Childhood Illnesses
Most every parent can relate to being worried or feeling frantic when trying to understand the best way to care... Read More ›
 10 Ways to Ensure Good Nutrition for Child Development
10 Ways to Ensure Good Nutrition for Child Development
Good nutrition is foundational in the healthy development of a child. Healthy food provides vitamins and minerals, fiber, protein, and... Read More ›
 Dr. Jenny Malcom Receives 2023 John L. Stevenson MD Award
Dr. Jenny Malcom Receives 2023 John L. Stevenson MD Award
Congratulations, Dr. Malcom! Dr. Jenny Malcom Pediatrician Pediatrician Dr. Jenny Malcom with Metropolitan Pediatrics has been named as this year’s... Read More ›
 The Importance of Sleep for Children
The Importance of Sleep for Children
Most people of every age have experienced the rejuvenating power of a good night’s sleep. But for our young ones,... Read More ›
 When to Call Your Pediatrician
When to Call Your Pediatrician
As a parent, you can always tell when your child isn’t feeling their best. But how do you know when... Read More ›
 Updated COVID-19 Vaccines Are Now Available
Updated COVID-19 Vaccines Are Now Available
Metro Pediatrics is now scheduling appointments for patients to receive the updated Moderna and Pfizer COVID-19 vaccine. Health authorities recommend... Read More ›